Unlike dry batteries, whose stored electrical energy can only be used once, accumulators have the immense advantage of reversibility, which translates into the possibility of a series of charge/discharge cycles. This means that they can undergo a series of charge/discharge cycles.
They are found in applications that may require more power, such as starting the thermal engines of road vehicles.
However, the reversibility that characterizes them also makes them much more interesting for modest powers. For example, in applications like portable devices, such as mobile phones or cameras.
General Characteristics
Basic Structure
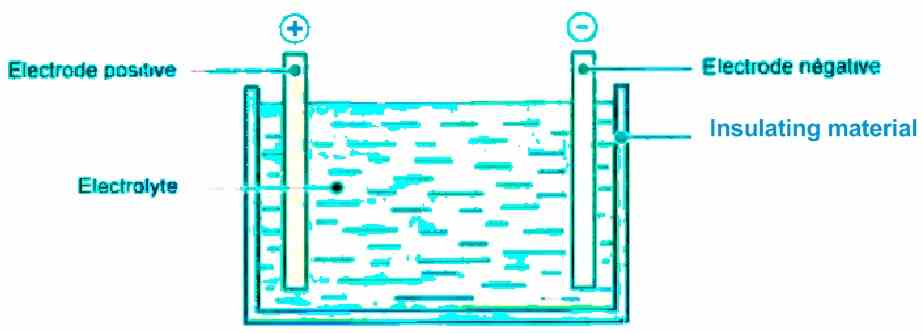
In general, an accumulator, commonly known as a "battery", consists essentially of:
- A positive electrode
- A negative electrode
- An electrolyte (liquid or solidified)
- A container enclosing the three elements mentioned above and can be completely sealed, as shown below.
Functioning
During charging of a rechargeable battery, an electrolysis occurs inside the electrolyte as the "charge" electric current circulates between the positive electrode (anode) and the negative electrode (cathode).
We will not go into the details of the electrochemical reaction that occurs on this occasion. This reaction essentially depends on the type of battery.
In a discharge situation, the reverse electrochemical reaction occurs. The battery provides energy in an external circuit. This results in a reversal of the current flowing within the electrolyte.
When the two electrodes reach the same electrochemical state, the battery is discharged and no longer has intrinsic electrical energy.
Voltage
This is an important characteristic. It is measured without load. Generally, the voltage available at the terminals of a basic element is only a few volts. This value also depends on the type of battery.
As it is often necessary to have a higher voltage value, such as 6 V, 12 V or even 24 V, the elements are connected in series inside the accumulator to form a "battery".
It is sometimes called EMF (electromotive force).
Capacity
The value of a battery's capacity is related to its size. Also called "electrical charge", it is expressed in Ah (ampere-hour) or mAh (1 Ah = 1000 mAh).
A battery with a capacity of 1 Ah is theoretically capable of supplying a current of 1 A for one hour.
Therefore, Ah is a unit of electrical quantity that official standards recommend expressing in coulombs (C).
The coulomb corresponds to a current of 1 A for 1 s. We can thus write the following correspondence:
1 Ah = 3600 C
Stored energy
As an energy, it is measured in joules (J) which is the energy corresponding to a current of 1 A under a voltage of 1 V for 1 s, or a power of 1 W for 1 s. It is often expressed in Wh or kWh. 1 Wh = 3600 J Since this notion includes the voltage, it is more significant than capacity.
Current
The current is expressed in amperes (A). This is the maximum value for which the battery can function without irreversible alteration or abnormal increase in its internal temperature.
Internal resistance
It is expressed in ohms (Ω). It is an internal resistance, rather harmful, which opposes the discharge current (see the diagram below). It transforms a portion of the energy returned by the battery into heat. It is a loss.
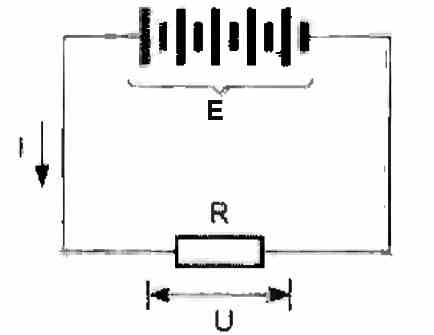
If this value of internal resistance is designated by "r", the theoretical voltage of the battery by "E", the external charging resistance by "R", the discharged current by "I", and finally the voltage measured at the terminals of the battery by "U", then the final equation can be written as given below:
E = U + (r x I) E = (R + r) x I
Charging current
This current is, of course, expressed in amperes (A). It is a value recommended by the manufacturer. It is often expressed as a ratio: that of the charging current to the capacity.
For example, if a battery is characterized by a capacity of 1 Ah and the recommended charging current is 200 mA, the ratio in question is 0.2 (0.2/1).
The purists will not fail to notice that this is an unorthodox ratio since two values expressed in different units are being compared.
Specific energy
This quantity determines the energy that the battery can provide relative to its mass. Therefore, it is expressed in Wh/kg or Ah/kg.
Another way to integrate the dimensional aspect, or more precisely, the volumetric aspect of the battery, is to state its volumetric density. It is expressed in Ah/m³ or, more commonly, in Wh/l (Wh/liter).
Different types of batteries
Lead-acid battery
This is the oldest type of battery and also the most polluting, as it contains sulfuric acid and lead. It is mainly used in road vehicles. However, there are also sealed lead-acid batteries with a gel electrolyte.
The basic element has a voltage of about 2.1 V. With six elements, a 12.6 V battery is obtained. Such a battery can provide high currents without damage, such as those required for starting a combustion engine.
Another advantage of lead-acid batteries is their low discharge rate, which is about 1% per month.
However, their energy density is low, and prolonged discharge can cause irreversible sulfation of the elements. For charging, it is preferable not to exceed 2.3 V per element.
For discharge, it is imperative not to go below 1.9 V. If the battery is unused, it must be stored charged. A lead-acid battery rarely lasts more than five years.
Nickel-cadmium batteries (Ni-Cd)
These are the most commonly used batteries in various portable devices, but they will be replaced in the long term by Ni-MH (Nickel-Metal Hydride) batteries due to the high pollution level of cadmium.
One of their qualities is their ability to provide relatively high discharge currents due to their very low internal resistance.
Characterized by a nominal voltage per element of 1.2 V, a sustained voltage is observed during discharge for nearly 70% of their capacity. Their recharge is also simple and presents large tolerances. However, rapid recharges should be avoided.
They also have some weaknesses, particularly a self-discharge rate that can exceed 20% per month.
However, their "memory effect" is undoubtedly the characteristic that needs to be taken into account.
That is why, during the first use, it is recommended to subject them to two or three complete charge/discharge cycles, which will provide them with maximum capacity.
It is especially important to avoid charging if the battery is not discharged, as this can result in memorization of the charge range between the remaining capacity and the maximum charge, leading to a decrease in capacity during subsequent charges. But be careful: fully discharging a battery of this type does not mean reaching 0 V.
The discharge is considered complete when the voltage per element reaches 1 V. It is better to store them discharged and perform charge/discharge cycles when they are put back into service. They can withstand up to two thousand cycles for a lifespan of up to eight years
Nickel-metal hydride batteries (Ni-MH)
Cadmium, a highly polluting element, has been removed from their composition. Introduced in the 1990s, these batteries are characterized by a significantly higher specific energy than Ni-Cd batteries, by at least 30%.
Additionally, they have a very low memory effect with much higher performance. Their storage does not pose any particular problems.
The main weakness lies in the difficulty of detecting the end of charge, as it is manifested by a slight inflection in the curve.
Their lifespan is also shorter than that of Ni-Cd batteries. Currently, there are high hopes for this type of battery for equipping future hybrid cars (combustion engine/thermal engine).
Nickel-zinc batteries (Ni-Zn)
This type has been developed for several decades. At first, it had the major disadvantage of a reduced number of cycles, which gave it a mediocre lifespan.
However, a new technology, whose work dates back to 2005, has now solved this problem. The performance of this type of battery, with a nominal voltage of 1.65 V per element, is far superior to that of Ni-Cd and Ni-MH batteries.
Its lifespan is comparable to that of Ni-Cd batteries, with a similarly reduced self-discharge phenomenon. Additionally, the "memory effect" is also much lower.
Lithium batteries
This type of technology distinguishes between batteries with:
- Lithium metal, in which the negative electrode is composed of metallic lithium
- Lithium-ion, in which lithium is in an ionic state through the insertion of an insertion compound
- Lithium polymer (Li-Po)
Batteries with lithium metal pose security problems related to the use of this material, and so those with lithium-ion have been preferred.
This type of battery currently occupies the first place in the market for portable electronics. Its strengths lie in a high energy density, zero "memory effect," and extremely low self-discharge.
The nominal voltage of an element is 3.6 V, and these batteries should never be discharged to less than 2.5 V.
In lithium polymer batteries, the electrolyte is a gel-like polymer. These batteries have advantages such as the ability to take on various shapes (e.g., in a badge) and can even be deposited on a flexible support. The voltage per element is slightly higher: 3.7 V.
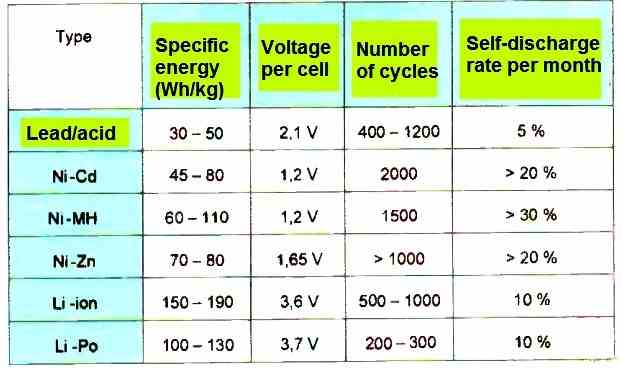
Summary
The above table summarizes, in a concise form, the main characteristics of these main types of rechargeable batteries.