In this article I have explained how vegetables can be used for making an organic battery, through an example of a practical potato battery experiment. Alessandro Volta was probably the first man to devise the method and idea of generating electricity from electrolytic solutions. According to his concept, two dissimilar metals when brought in contact with an electrolytic solution would initiate electron movement across the two metals, joined externally together using a conductor.
Introduction
All living being including plants are made up of fluid material which may be typically considered as an electrolyte.
As per the above concept, if two dissimilar metals are inserted through a plant or any living being body, should start the conduction of electrons constituting the generation of electricity.
All types of batteries, even the modern SMF types are based and work on this principle for generating electricity. However these are immensely sophisticated and efficient and therefore are able to produce sustained amounts of high current, for much longer periods of time, occupying very little space.
In this article we will try to analyze the above explained facts regarding generating electricity from vegetables and fruits. Since these are generously filled with electrolytic material become ideally suited for the required experiments.
In the first experiment we are using potatoes for generating DC from it, let’s learn the entire procedure and the materials required for conducting it:
Making a Potato Battery
You will require the following materials for the proposed experiment:
25 nos. medium sized fresh potatoes.
25 pairs of dissimilar metal pieces of any shape, preferably having sharper edges, so that they may be easily cut through the potato for making the necessary contacts.
25 nos of small lengths of wire, cut into suitable lengths and stripped at the edges for the required connections,
An LED, red in color is more suitable, as its more easily visible even during daytime and requires minimum amount of voltage for illuminating brightly.
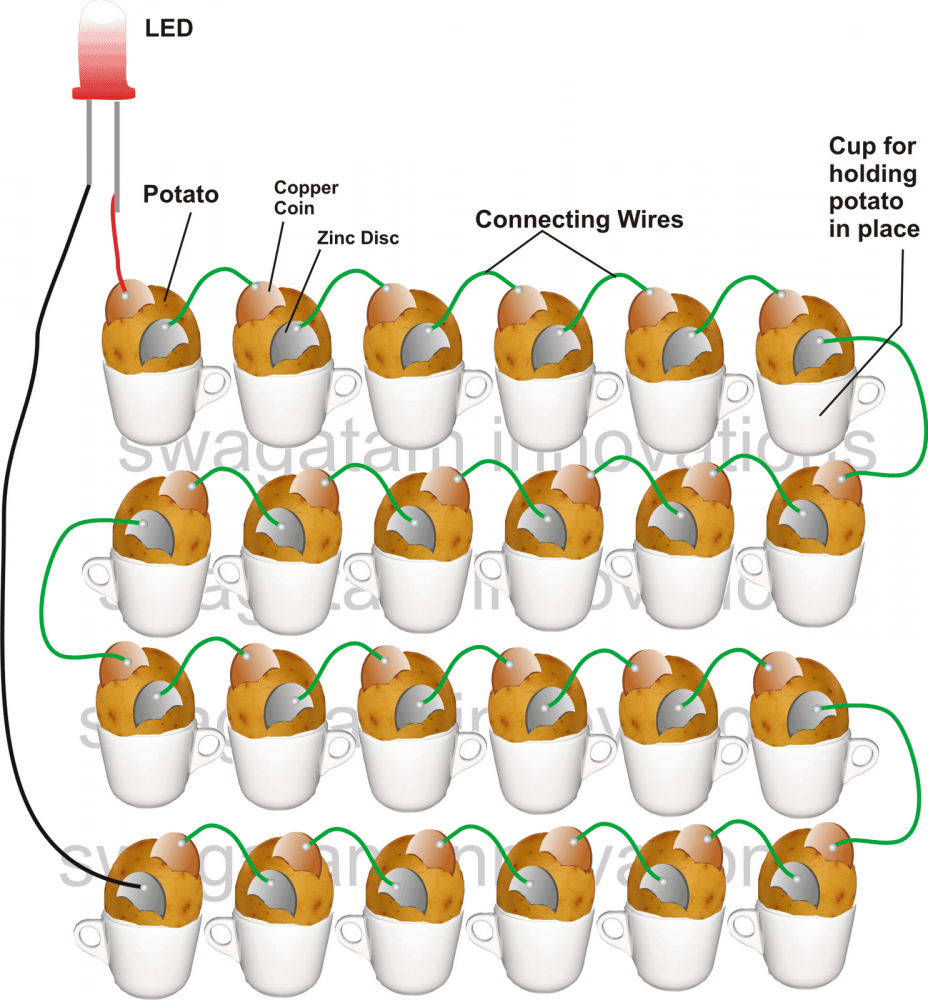
How to Assemble the Potato Battery Circuit
Clean the potatoes with a cloth so as to remove any dust particles or mud from its surface.
Also make sure the metal pieces are also cleaned so that it becomes free from any oxidized film deposits or any corroding layer. Use a sand paper for scrubbing the metals and for providing it a polished look.
Arrange the potatoes in a line by securing each one of them in some kind of container, for example inside cups or glasses as shown in the figure.
Start inserting the metals alternately, beginning from the first potato to the last one as specified in the figure.
Using a soldering iron, connect the alternate metal strips from one potato to the other with the given pieces of wires.
Finally you will have the two ends of the metals from the two extreme potatoes, free and open.
Terminate wires from these extreme ends using rather longer lengths of flexible wires and connect their ends to an LED, just as shown in the figure.
If everything is done correctly as described in the illustration, your LED should instantly start showing a pretty bright glow, indicating the reactions between the metals and the electrolyte inside the potato.
Using Lemons for Generating Electricity:
Lemons as we all know are acidic with their content and experiments have shown that acids react more violently with a given set of dissimilar metals in contact with them and therefore are able to generate electricity more efficiently.
For conducting the above experiment using lemons, we would require half the number of lemons than the amount required with potatoes.
Therefore we may need just 12 lemons for getting the above results.
The procedure remains the same, as above, and hopefully the results also will be the same if done exactly as specified in the diagram.
The above experiment can be repeated and verified by using different fruits and vegetables and also by using different sets of metals.
Preferably, copper and zinc produce the best possible results; however you may well try other sets of metals like copper and iron, copper and aluminum, Iron and aluminum and so forth.
Calculating the Parameters
Voltage Output
- Equation: V = Ecathode - Eanode
- Explanation:
- V: Voltage output of the battery (volts)
- Ecathode: Reduction potential of the cathode material (e.g., +0.34 V for Cu²⁺/Cu)
- Eanode: Oxidation potential of the anode material (e.g., -0.76 V for Zn/Zn²⁺)
- Example: For a zinc-copper potato battery:
- V = 0.34 - (-0.76) = 1.1 V
Current (Ohm's Law)
- Equation: I = V / R
- Explanation:
- I: Current (amperes)
- V: Voltage output (volts)
- R: Resistance in the circuit (ohms)
Power Output
- Equation: P = V * I
- Explanation:
- P: Power (watts)
- V: Voltage output (volts)
- I: Current (amperes)
Series Connection of Cells
- Equation: Vtotal = V1 + V2 + V3 + ...
- Explanation:
- Vtotal: Total voltage
- V1, V2, V3, ...: Voltage of individual cells
Energy Stored
- Equation: E = P * t
- Explanation:
- E: Energy (joules)
- P: Power (watts)
- t: Time of operation (seconds)
Internal Resistance of the Potato
- Equation: R_internal = (VOC - Vload) / I
- Explanation:
- Rinternal: Internal resistance of the potato (ohms)
- VOC: Open-circuit voltage
- Vload: Voltage under load
- I: Current through the circuit
hi sir this is nsgendra. i would like to know abt somemore information about the electricity ..c my question is wheather is it possible to conduct any devices without ground
hi, no, without ground it may be impossible to operate any electrical equipment.
Can you post the explaination chart of the experiment shown above